CTCBIOPSY® A10 Product Feature
Easy Operation:
-- High automation, 10 mins to complete CTC capture and enrichment of 5ml of whole blood.
-- Nano-microscreen technology, no reliance on specific cell marker, suitable for all types of tumors.
Exclusive CTM:
-- No lysis of red blood cells or pre-centrifugation to preserve the CTM intact, direct observation under a
microscope.
-- Pioneer in accurate classification of CTM in China, screen patients containing neutrophil CTM with the
highest risk of metastasis.
Adequate Data:
-- The first CTC device certified by NMPA in 2015, with international patent.
-- Over 30,000 clinical samples' validation in over 100 Grade-A tertiary hospitals in China.
-- Autonomous downstream analyses, such as IF, IHC, FISH and sequencing to meet the requirements of clinical
research.
Superior Performance of CTC-BIOPSY®-A10
Optimized isolation technology by size of epithelial tumor cells (ISET) can accurately and reliably capture
abnormal cells in blood, providing an efficient solution for tumor screening.
Automated Device
High-precision peristaltic pump with negative pressure
High-precision pressure sensor
Automatic control and simulation
|
Easy Operation
Friendly operation interface
Sorting of samples with one click
Real-time display of blood sample processing
|
 |
Authoritative Identification
Norms for identification of cell morphology
formulated by authoritative pathology experts
|
Quick Separation
No reliance on cell marker for separation
Single sample isolation time <10 mins
|
CTC-BIOPSY®-A10 Patented ISET Technology
Basic principle: ISET technology uses the size and deformability of tumor cells to achieve separation, then use
cell morphology for identification.
With good light transmission, second-generation nano polymer material is resistant to various staining reagents,
allowing direct observation of cells in visible light.
Nano-microsieve technology broke the foreign monopoly, high-precision lithography machine for laser lithography.
13mm micro sieve membrane has 160,000 nano micro sieves with an aperture of 8um to maximize the sorting of blood
cells and tumor cell enrichment.
Uniform hole spacing
|
Excellent light transmission of membrane
|
Uniform aperture
|
Resistance to various staining reagents
|
CTC-BIOPSY®-High Detection Rate of CTM
CTC detection data of the CTC-BIOPSY® system in 2018-2020 showed an average CTC detection rate for each tumor is
80.90%, CTM (Circulating Tumor Microemboli) detection rate is 12.52%, and the ratio of CTM to total CTC detection
was consistent with study data from Nature in 2019.
| Type |
Total cases |
Detected Qty |
Detection rate |
Mean |
Median |
| CTC |
9304 |
7527 |
80.90% |
5.154 |
3 |
| CTM |
1165 |
12.52% |
0.7813 |
0 |
| CTCs |
7686 |
82.61% |
16.42 |
3 |
CTC-BIOPSY®-Accurate Classification of CTM
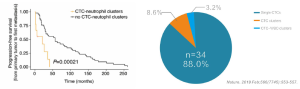
CTC neutrophil clusters, representing a critical weakness in the metastatic process, the link between neutrophils
and CTC drives the progress of cell cycle in the blood flow and expands the metastatic potential of CTC.
CTCBIOPSY® is the only NMPA approved detection system in China, it can efficiently capture CTMs containing
neutrophils, which can be seen directly with the eye under ordinary optical microscopes. Patients with a higher
risk of metastasis can be accurately screened for clinical diagnosis and treatment.
CTCBIOPSY® Systematically Detect CTM Containing Neutrophils: